Games
Game - Atomic Labs
Get hands-on with science investigations in Atomic Labs. Experiment and put your science skills to the test using Bunsen burners, test tubes and much more.

The particle model of matter
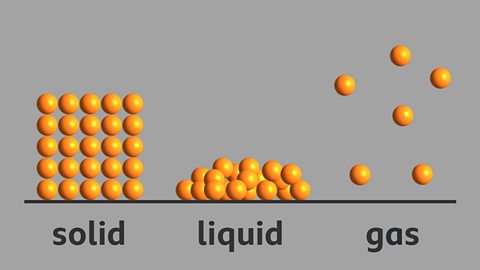
Solids, liquids and gases
Particles can be atoms, molecules or ions. Particles behave differently in solids, liquids and gases. The particle model explains the differences between solids, liquids and gases.

Changes of state
Solids, liquids and gases change state when they are heated or cooled. Processes such as evaporation and boiling change the state of substances
Gas pressure
Pressure in gases is caused by particles colliding with the walls of the container.

What is water?
Learn about water in this KS3 guide.

Atoms, elements and compounds
Elements, compounds and mixtures
Learn about elements, compounds and mixtures in this KS3 Chemistry guide from 91»»±¨ Bitesize.

Atoms and molecules
Atoms are the building blocks of everything. Atoms can form strong bonds with each other, making molecules.

Symbols and formulae
Letters from the alphabet are used to represent chemical elements. An element’s chemical symbol is a single capital letter or a capital letter followed by a lower case letter.
What is carbon?
Find out about the structure and properties of the element carbon.

What are elements?
A visual explanation of chemical elements.

What is oxygen?
A visual explanation of oxygen.

What is nitrogen?
A visual explanation of nitrogen.

What is hydrogen?
A visual explanation of hydrogen.

Pure and impure substances
Evaporation
Either evaporation or crystallisation can be used to separate a solid solute from a solution. Learn more in this KS3 Chemistry guide from Bitesize.
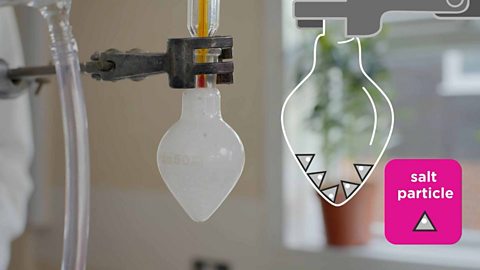
Distillation
Distillation is a separation technique is used to remove a solvent from a mixture and keep it rather than it mixing with the air and being lost.
Chromatography
Chromatography can be used to separate a mixture of soluble substances, like the pigments in ink.

Pure substances
Most materials that we use are mixtures, and just a few are pure elements or pure compounds. In chemistry, a pure substance is a single substance made of only one type of particle.

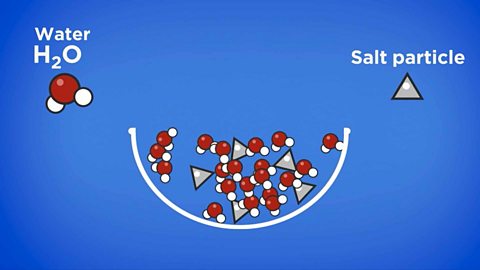
Dissolving
A solution is made when a solute dissolves into a solvent. If a substance can dissolve into a solvent, it is soluble. If it cannot dissolve, it is described as insoluble.

Diffusion
Diffusion is the movement of a substance from an area of high concentration to an area of lower concentration.

Filtration
Filtration is used to separate an insoluble solid from a pure liquid or a solution.

Chemical reactions
Introduction to chemical reactions
Chemical reactions make new chemicals. Atoms are rearranged during a chemical reaction, but the number of atoms does not change.

Oxidation
In an oxidation reaction, a substance gains oxygen atoms. Learn more in this KS3 Chemistry guide from Bitesize.

Catalysts
A catalyst is a substance that speeds up a chemical reaction. Catalysts are useful because they do not get used up during a reaction.

Exothermic and endothermic reactions
Exothermic reactions release energy into the surroundings, so they usually feel hot. Endothermic reactions are the opposite.

Writing word equations
Chemical reactions can be represented by word equations. Learn more in this KS3 Chemistry guide from Bitesize.

Writing symbol equations
Chemical reactions can be summarised using symbol equations. Learn more in this KS3 Chemistry guide from Bitesize.

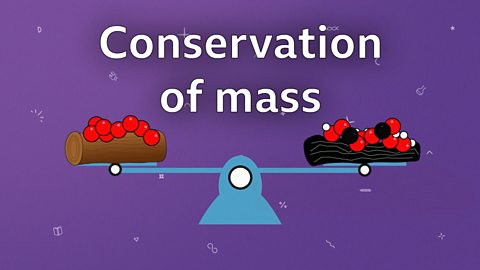
Conservation of mass
During chemical reactions or a change of state, no atoms are created or destroyed. The total mass of chemicals before and after a reaction remains the same.
What is combustion?
Combustion is another name for burning. In a combustion reaction, fuel is burned and reacts with oxygen to release energy.

Thermal decomposition
Thermal means heat. Decomposing is the process of breaking down. Thermal decomposition is a chemical reaction that happens when a compound breaks down when heated.

Introduction to displacement reactions
In a displacement reaction, a more reactive element replaces a less reactive element from its compound.
How to make CO‚ÇÇ
A simple, step-by-step, visual guide showing you how to make CO‚ÇÇ.

How to make bread
A simple, step-by-step, visual guide showing you how to bake bread.

Acids and alkalis
The pH scale
The pH scale shows how acidic a substance is. Learn what pH means and how it is measured in this KS3 chemistry guide from 91»»±¨ Bitesize.

Neutralisation reactions
Acids and alkalis react together in neutralisation reactions which produce salts and water. The salt name can be found from the name of the acid and alkali.

Reactions of metals with acids
Acids react with some metals to produce salts and hydrogen. The salt name can be found from the name of the acid and metal.

Acids and bases
Acids, bases and alkalis are found in the laboratory and at home. Acids and bases can neutralise each other. A base that can dissolve in water is also called an alkali.

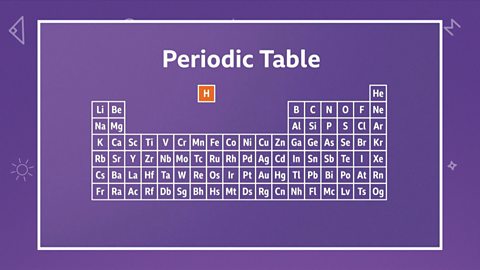
Periodic table
Physical and chemical properties
All substances have properties. These describe how a subject looks and behaves. Substances have both physical and chemical properties.

Developing the periodic table
The periodic table was developed by grouping elements with similar properties. This, together with a later arrangement by atomic weight, led to a repeating pattern of properties.

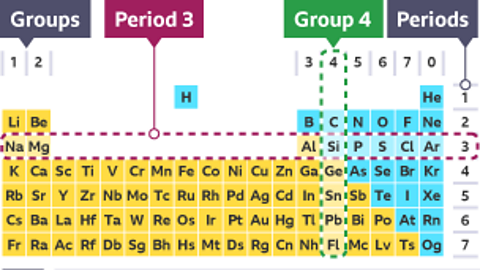
The modern periodic table
The periodic table is a way of organising the elements which is used by scientists to group elements with similar properties. It has a unique arrangement of rows and columns.
Metals and non-metals
Metals make up most of the known elements with non-metals making up the rest. They tend to have opposite properties. Metals are mostly solids;, non-metals liquids or gases.

Metal and non-metal oxides
Metals and non-metals can be heated in oxygen to make compounds called oxides.

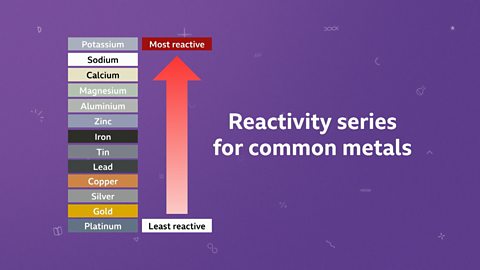
The reactivity series
Making a reactivity series
Metals react differently. Some are very reactive and others are unreactive.

Extracting metals
Metals mostly occur as compounds in rocks and minerals and must be extracted before they can be used.

Displacement reactions
A metal higher up in the reactivity series will displace a less reactive metal from its compound. These displacement reactions can be predicted from the reactivity series.

Materials
Ceramics
Ceramics are made from soft substances, which when heated become hard and brittle.

Polymers
Polymers can be natural or synthetic. Synthetic polymers are known as plastics.

Composites
Composite materials are made from two or more different types of material by a chemical process.

The Earth and atmosphere
Atmospheric pollution
Many human activities release polluting gases into the atmosphere, such as farming animals for food, burning fossil fuels and deforestation.

Resources from the Earth
Human activities use resources from the Earth. Many of these resources are finite, they will eventually run out.

Composition of the atmosphere
The air in the atmosphere is a mixture of gases. Most of the atmosphere is nitrogen (80%) with around 20% oxygen and other trace gases.

What is methane?
A visual explanation of methane.

What is carbon dioxide?
Find out all about carbon dioxide

Structure of the Earth
The Earth has a layered structure made up of the crust, the mantle, the outer core and the inner core. These layers each have different structures and properties.

Rock types
There are three types of rocks on Earth, igneous, metamorphic and sedimentary. These are formed in different ways and have different properties.

The rock cycle
Rocks are constantly due to different processes. This means rock types are changing between the three different rock types over millions of years. This is called the rock cycle.

Working scientifically
Working safely in the lab
How can you work safely in the lab? What are the various steps needed in order to do so? How to spot risks, hazards and understand hazard symbols.

Variables
Find out why variables are important in an experiment, including control variables, independent and dependent variables.

Writing a hypothesis and prediction
A hypothesis is an idea about how something works that can be tested using experiments. A prediction says what will happen if the hypothesis is correct.

Planning an experiment
Developing a method is an important part of planning an investigation and it is important to evaluate the validity of each step leading up to the conclusion.

Maths skills for science
Scientific equations and formulae are used to work things out in science. Using standard units means scientists around the world can work together better.

Drawing scientific apparatus
Use diagrams to show how scientific apparatus is set up and how to draw them in 2D. Common practical techniques that use apparatus include filtration, evaporation and distillation.

Observation and measurement skills
Calculating averages from data is usually more accurate than using one result. Using a table ensures that data is recorded in an organised way.

Types of data
Types of data include continuous, discrete and categoric. Data can be presented in a number of ways, which depends on the type of variable and the uses.

Graphs and charts
Different types of graphs and charts are used to show results. They need to be drawn and labelled correctly. Scientists use patterns in the data to reach a conclusion.

Conclude and evaluate
A conclusion sums up what has been found out during an investigation. It should be clearly structured and explained using scientific knowledge.

Bias in science
Scientific studies can be biased; it's important to recognise when this is the case. Scientists review each other's conclusions from new research.

Crude oil and fuels
What is methane?
A visual explanation of methane.

How science works
Franklin v Hodgkin: Who was the greatest scientist?
Find out about the two great scientists, Franklin and Hodgkin.

Links
- External linkExternal link
- External linkExternal link
- SubscriptionSubscription
- External linkExternal link
- External linkExternal link
- External linkExternal link
- SubscriptionSubscription