Key points
- Almost everything is made of particles.
- Particles can be atoms, molecules or ions.
- Particles behave differently in solids, liquids and gases.
- The particle model explains the differences between solids, liquids and gases.
Water exists as a solid, liquid and as a gas. What name is used for solid water and gaseous water?
Solid water is ice. Gaseous water is steam.

Video
Watch this video to see a model of how particleA single piece of matter from an element or a compound, which is too small to be seen. Particles can be atoms, molecules or ions. are arranged in solids, liquids and gases.
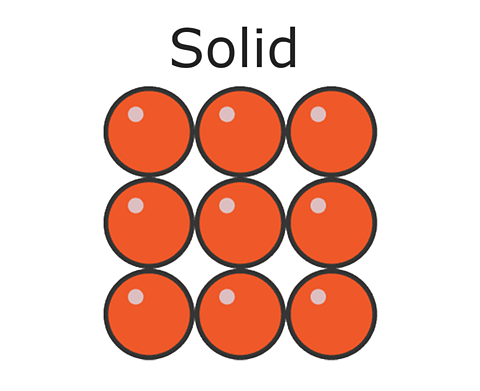
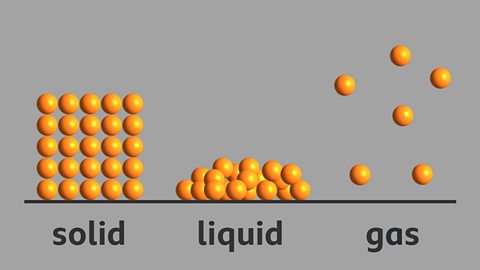
Solids, liquids and gases. In a solid like this brick, the particles are regularly arranged touching their neighbours and move only by vibrating. This explains why solids have a fixed shape.
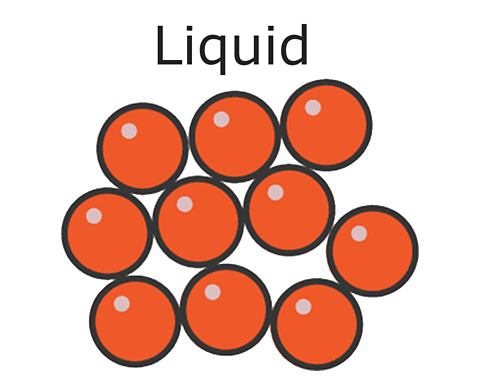
In a liquid like water, the particles are randomly arranged. They move freely over each other, a bit like marbles in a bucket.This is why liquids can be poured.
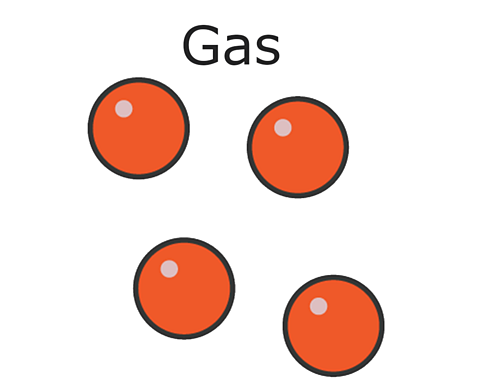
In a gas, like helium, particles are widely spaced apart and move quickly and random directions. They have no fixed volume and fill the space of their container. They travel in straight lines until they hit something, like the walls of the container they're in, or each other.
Describe the arrangement and movement of the particles in a solid, liquid and gas.
In a solid, the particles pack together tightly in a neat and ordered arrangement. The particles are held together too strongly to allow much movement but the particles do vibrate.
In liquids, particles are quite close together and move with random motion throughout the container.
In gases the particles move rapidly in all directions, frequently colliding with each other and the side of the container.
Solids
Lots of materials are solid, such as paper, bricks, wood, metal, and ice.
The particles in solids are very close together, therefore they cannot usually be compressed or squashed. forceIn chemistry, 'force' is used to describe the attraction or repulsion between two particles. of attraction between the particles hold them together and keep them in place.
The particles in solids are arranged in a regular way. The particles in solids move only by vibrating about a fixed position. This gives solids a fixed shape and means that they cannot flow like liquids.
The hotter a solid gets, the faster its particles vibrate. This means that solids expand when they are heated.
Did you know?
- When substances are heated and expand, their particles do not get any bigger.
- When heated, the particles begin to vibrate more which causes them to move slightly further apart.

By looking around, how many examples of solids can you see, touch, hear or feel?
There are probably lots. Some common examples could be clothing, sometimes made from cotton or polyester. A building is made from solid materials like glass, bricks and steel.

Liquids
There are many different liquids such as; water, oil, fruit juice, and many others.
The particles in liquids are arranged in a random way, and are close together, touching many of their neighbours. There are some gaps, but liquids cannot usually be compressed or squashed.
The particles of a liquid have enough energy to break free of some of the forces of attraction between the particles. So particles in liquids can move around and can move over each other, allowing liquids to flow and be poured.

In a diagram of the particles in a liquid, make sure that they are mostly touching but randomly arranged.
If there are large spaces between the particles, you would be able to compress the liquid.
Sand can be poured, but it is definitely a solid. Why is this?
Sand is made from tiny grains, and even though these can roll over each other, each grain of sand has a fixed shape. You can pour marbles too, but they are also definitely solid.
Gases
There are lots of different gases, such as the air we breathe or the helium used to fill balloons.
The particles in gases are widely spaced and randomly arranged, meaning they can be easily compressed or squashed.
The particles in a gas have enough energy to overcome the forces of attraction between the particles, so are free to move in any direction. They move quickly in straight lines, colliding with each other and the walls of their container.

There are large spaces between the particles of a gas. These spaces between particles are empty, there is nothing in them.
Why is helium gas used inside party balloons?

Helium gas is lighter than the mixture of gases that make up air. The lighter helium gas is trying to move above the air making the balloon rise up.
The particle model of matter
Almost everything is made of particles, with the exception of electromagnetic waves, such as light and X-rays.
A particle is a single piece of matter from an element or a compound, which is too small to be seen, even with the most powerful microscope that you could find in a school science lab. Particles can be atomThe smallest particle of an element. We often think of atoms as tiny spheres, but in fact they are made from smaller particles called protons, neutrons and electrons.,moleculeTwo or more atoms which are strongly bonded together. The smallest particle of a substance that has all of the physical and chemical properties of that substance. or ionAn atom or molecule that has either a positive or negative electrical charge..
What are models?
modelA simple representation of something or a way of explaining something complicated. A model may be an equation, a diagram or an analogy that helps explain a scientific idea. are used in science to help explain scientific concepts. The particle model is the name for the diagrams used to draw solids, liquids and gases.
In the model, the particles are shown as circles or spheres. However, the particles in ice, liquid water and steam look the same because they are all water, but in different states of matter.
Why do we use the particle model in science? The particle model is not exactly the same as real life. Can you think of a way in which it is different?
Scientists use models to show things that are too small to see, like atoms or cells. Sometimes they use models of things which are much bigger, like the solar system.
Can you think of a scientific model which is life-sized?
You might have seen a model of a human skeleton or the organs within the human body at school, which are life-sized examples of a scientific model.
Working scientifically

Shear-thickening liquid
Shear-thickening liquid is an unusual liquid that can be slowly poured but acts like a solid if quickly struck. Try making this at home.
You will need six spoonfuls of cornflour or custard powder and three spoonfuls of water.
- Pour the cornflour into a container and slowly add water.
- Mix gently. It is hard to mix the cornflour and water.
- Stir the cornflour slime very slowly using the spoon. Then stir it quickly. Which is harder?
- Using two fingers, hit the cornflour slime very quickly. What happens?
Now slowly place your fingers into the slime. What is different? - Pick up a little slime and roll it into a ball with your hands. How does it feel?
Now stop rolling. How does its behaviour change? - Try adjusting the amounts of cornflour and water and notice the effects.

Variables
Scientific investigations often involve things that can be changed, known as variables. Scientists often want to find out if changing one variable will make a difference to another variable.
What are the variables in the shear-thickening liquid activity?
Some variables are whether you use cornflour or custard powder, the number of spoonfuls of water, and the number of spoonfuls of cornflour or custard powder.
Try this activity five times but change one variable each time. Keep the others constant. See how this affects the properties of the mixture you have made.
For example, make up two samples of shear-thickening liquid with the following variables.
The control variable is kept the same during the experiment. As the control variable, in both samples, use the same number of spoonfuls of cornflour.
The independent variable is changed during the experiment. In each sample, use a different number of spoonfuls of water as the independent variable.
The dependent variable is tested or measured during the experiment. Measure the dependent variable by observing how quickly the mixture flows down a sloping surface.
Find out more about variables.
Test your knowledge
Play the Atomic Labs game! gamePlay the Atomic Labs game!
Try out practical experiments in this KS3 science game.

More on The particle model of matter
Find out more by working through a topic
- count2 of 4
- count3 of 4

- count4 of 4
