Key points
Carbon dioxide is a compoundA pure substance made from two or more elements which are chemically bonded in a fixed ratio. . Its molecules consist of one carbon atom joined to two oxygen atoms.
In the atmosphere, carbon dioxide is a greenhouse gas Gases such as water vapour, carbon dioxide, and methane in the EarthÔÇÖs atmosphere that trap heat..
We use carbon dioxide to make drinks fizzy and keep foods fresh.
The Chemistry of carbon dioxide
Get an introduction to the chemistry of carbon dioxide from a development engineer.
Structure and properties
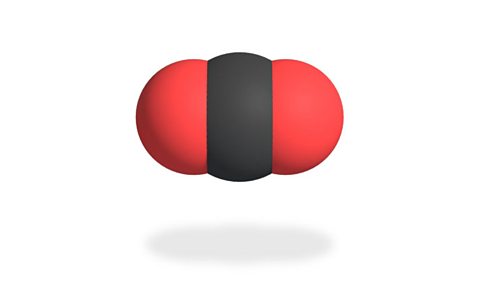
At room temperature carbon dioxide is a colourless and odourless gasOne of the three states of matter. Gases, like oxygen or helium, do not have a fixed shape and can expand to fill their container..
The carbon dioxide moleculeTwo or more atoms which are strongly bonded together. The smallest particle of a substance that has all of the physical and chemical properties of that substance. is made up of one carbon (C) atom joined to two oxygen (O) atoms. This means it has a chemical formula is ░ń░┐Ôéé.

░ń░┐Ôéé in the atmosphere
The amount of carbon dioxide in the atmosphereThe atmosphere is the layer of gases surrounding a planet. is maintained through a balance.
Processes that increase the amount of ░ń░┐Ôéé in the air include:
- combustionThis is the scientific word for burning. It is where a substance reacts with oxygen from the air and transfers energy to the surroundings by heating and light. of petrol and diesel in cars
- respirationThe chemical process that occurs inside the cells of most living things, when glucose reacts with oxygen to produce carbon dioxide and water. of living things
Processes that decrease the amount of ░ń░┐Ôéé in the air include:
- photosynthesisA chemical process used by plants to make glucose and oxygen from carbon dioxide and water, using light energy. Oxygen is produced as a by-product of photosynthesis. Algae subsumed within plants and some bacteria are also photosynthetic. by plants
- dissolveThe process when a solute is mixed with a solvent and the solute breaks into much smaller particles and spreads out. in sea water

Carbon dioxide makes up less than 1% of the atmosphere; however, it is an important greenhouse gas.
This means that its molecules in the atmosphere absorb heat radiation from, keeping the Earth warmer than it would otherwise be.
For the past 100 or so years, carbon dioxide has been added to the atmosphere more quickly than it is removed. The extra carbon dioxide contributes towards global warmingThe rise in the average temperature of the Earth's surface..
Global warming leads to climate change, including more frequent droughts and stronger storms.
Test your knowledge
Carbon dioxide quiz
Find out how much you know in this quick science quiz!
Play the Atomic Labs game! gamePlay the Atomic Labs game!
Try out practical experiments in this KS3 science game.

More on The Earth and atmosphere
Find out more by working through a topic
- count6 of 8

- count7 of 8

- count8 of 8

- count1 of 8
