Key points
- All substances have properties. These describe how a substance looks and behaves.
- There are two types of properties: physical and chemical.
When liquid water boils, it produces steam. Is this a physical or chemical reaction?
It is a physical reaction because it changes state.

Video
Watch this video about how physical and chemical properties affect which materials we use in everyday life.
While you're watching, try to look out for properties which are opposite to each other.
I’m going to find out why we make frying pans out of metal, and why the case around electrical plugs out of plastic, but not the other way round.
It’s because of the physical properties of the materials. Physical properties are the basic features of a material that affects how it behaves in different situations.
Metal is a good conductor of heat, and has a high melting point, making it good to cook with.
Whereas the plastic of the handle doesn’t conduct heat so well, it’s an insulator. And plastic has a lower melting point too.
But plastic is a good electrical insulator, meaning we can touch the outside of electrical plugs and wires without getting an electric shock. The electric current can flow through the inner metal part of the wire to light the lamp. Metal is a good electrical conductor.
Both the metal and the plastic can be easily shaped into long flexible wires - we say they’re ductile.
Whereas other materials, like the glass bulb of this lamp, are brittle. They’re more easily broken.
Sometimes, we choose the right material based on its chemical properties - which describe how reactive it is. Like the stainless steel metal in cutlery, which doesn’t react with food and remains shiny after washing.
Whereas normal steel will rust when exposed to air and water.
And wood will react with oxygen if heated.
It’s important to pick the right material for a job based on its chemical and physical properties.
I don’t think a plastic frying pan would be very useful!
Why are electrical cables coated in plastic?
The plastic acts as an electrical insulator, meaning we can touch the outside of electrical plugs and wires without getting a shock. Plastic is also ductile, which means it can stretch and bend with the wires.
Properties
A propertyA quality, or characteristic of something. Physical properties of matter are measurable, for example melting point or boiling point. is something that describes how a substance behaves. There are 2 types of properties:
chemical propertyThe way an element or compound reacts with other chemical substances. - chemical changes happen when chemical reactions occur. They involve the formation of new chemical elements or compounds.
physical propertyA property of an element or compound which can be directly observed or measured. For example, melting point, electrical conductivity, appearance at room temperature. - in a physical change, a substance simply changes physical state. For example, from a solid to a liquid.
There are almost 100 metals in the periodic table. Knowing about a substance’s properties is important for making choices about materials for particular uses.

Did you know?
Most cooking pans are made from aluminium and steel. This is because a property of aluminium and steel is that they are excellent conductorA material which allows heat or electricity to move easily through it. of heat. This means that thermal energy from the stove transfers efficiently into the food we cook.

True or false? Water changes from a liquid to a gas during evaporation, which is an example of a chemical change.
False! Evaporation is a physical change.
This is because physical changes occur when a substance simply changes physical state, such as from a liquid to a gas.
Physical properties
Physical properties can be seen and can usually be measured quite easily in an experiment. Here are some examples:
- By applying force to an object you can observe it's physical properties. If something is brittleSomething which is brittle is easily broken or shattered. the object may snap, or if it is flexibleA substance is flexible if you can bend it and it returns to its original shape after the bending force is removed. the object will bend.
- By observing a solid's melting pointThe temperature at which a pure substance melts from a solid into a liquid. For example, the melting point of pure water is 0°C. The melting point is also the temperature at which a liquid will freeze to a solid., or a liquid's boiling pointThe temperature at which a pure substance boils from a liquid into a gas. For example, the boiling point of pure water is 100°C. The boiling point is also the temperature at which a gas will condense into a liquid. these physical properties can be measured.
This diagram shows the physical changes that take place when water changes state.
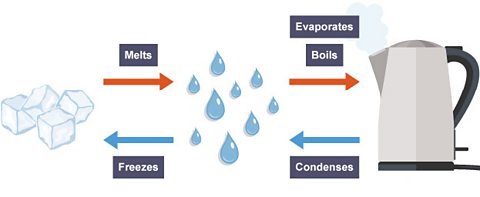
Water goes through different physical changes. Are any of these physical changes permanent?
No, water doesn't permanently change physical state.
Water that has been boiled and changed state to a gas (evaporation) can cool down and return to its liquid state (condensation).
Chemical properties
Chemical changes happen when chemical reactionWhen chemical bonds are broken and made between atoms, so that new substances (compounds or elements) are made. occur. However, the properties cannot be learnt just by looking at, touching or taking a simple measurement of the substance.
Examples of how we can measure chemical properties include:
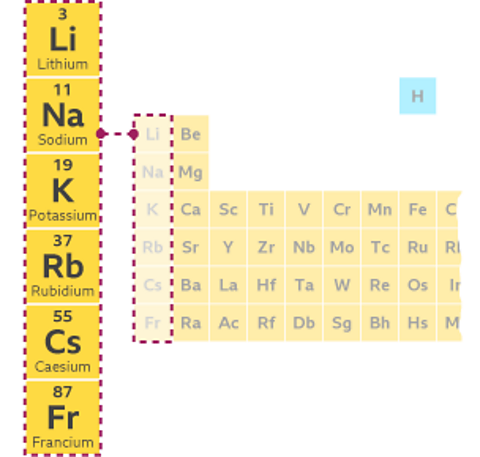
- Observing how elements react with air.
For example, the Group 1 elements react with moist air. They are stored in oil to stop air and water vapour coming into contact with them. Moist air reacts with potassium to form potassium oxide. This creates a layer over the surface of the metal.

Find out more about the Group 1 elements in this guide.
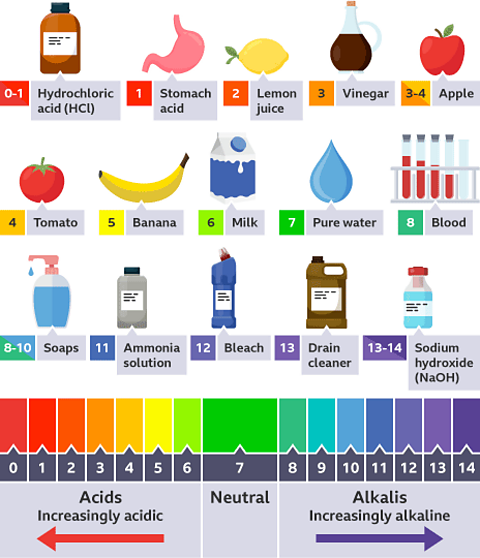
- We could measure the pH of a liquid.
The pH scale shows how acidic a substance is. It can be measured using a pH meter which gives a numerical value. pH can be also be measured using an indicator and comparing the colour with a comparison chart.

Find out more about the pH scale in this guide.
Test your knowledge
Quiz
Play the Atomic Labs game! gamePlay the Atomic Labs game!
Try out practical experiments in this KS3 science game.

More on Periodic table
Find out more by working through a topic
- count2 of 6

- count3 of 6
- count4 of 6

- count5 of 6
