Structures, trends, chemical reactions, quantitative chemistry and analysis
Atomic structure - (CCEA)
Scientists’ ideas about atoms have changed over time. Today, they agree that atoms have a positively-charged nucleus made of protons and neutrons, and negatively-charged electrons that orbit the nucleus in shells.
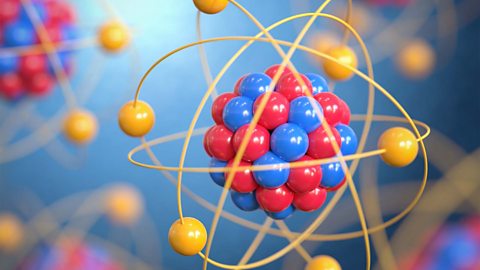
Bonding - (CCEA)
Atoms and ions bond with each other in three main ways – ionic bonds, covalent bonds and metallic bonds. Different types of bonds form different types of structures – lattices and molecules.
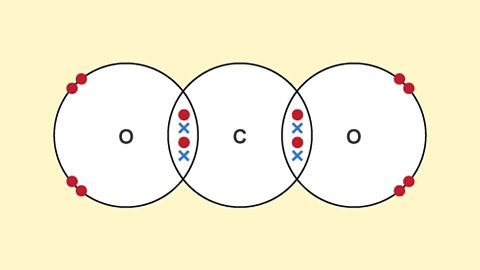
Structures - (CCEA)
Ionic bonding holds ions together in a giant lattice. Covalent bonds create simple molecules or giant covalent structures. Different types of bonding give a substance different properties.

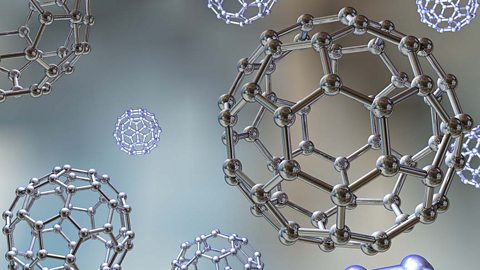
Nanoparticles - (CCEA)
Nanoparticles have large surface to volume ratios. This gives them interesting properties.
Symbols, formulae and equations - (CCEA)
Symbols, formulae and equations help chemists to explain chemical reactions in detail.
The periodic table - (CCEA)
The periodic table helps to categorise the known elements and make predictions about ones that we haven’t yet discovered.

Quantitative chemistry - (CCEA)
Chemists use relative atomic masses and relative formula masses to carry out mole calculations.

Acids, bases and salts - (CCEA)
Many chemicals are acidic, neutral or alkaline. We can distinguish one from another using indicators. Acidity and alkalinity are measured on the pH scale. A salt is formed when an acid is neutralised by an alkali.

Chemical analysis - (CCEA)
Most elements are rarely found in their pure form. They are found chemically combined with other elements in compounds. Compounds are often found mixed with other compounds. Mixtures may be separated and analysed.

Solubility - (CCEA)
Solubility is a measurement of the maximum mass of a substance which will dissolve in 100 g of water at a particular temperature. The solubility of solids and gases in water varies with changes in temperature.

Links
- External linkExternal link
- External linkExternal link
- SubscriptionSubscription
- External linkExternal link
- External linkExternal link
- External linkExternal link
- SubscriptionSubscription
- External linkExternal link