Prescribed practicals
1. Determine the mass of water in hydrated crystals
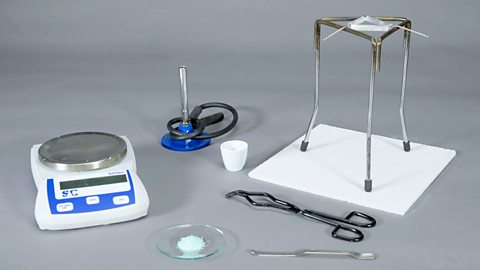
In this experiment you will heat hydrated iron(II) sulfate crystals. By carefully heating the crystals and recording mass measurements, we can calculate the mass of water in the supplied crystals.
2. Investigate the reactions of acids
Investigating the reactions of acids, including temperature changes that occur.

3. Investigate the preparation of soluble salts
Making a soluble salt from a base and making a soluble salt from an alkali.

4. Identify the ions in an ionic compound
Identifying the ions in an ionic compound using chemical tests.

5. Investigate the reactivity of metals
Determining the relative reactivity of metals.

6. Investigate the rate of reaction
Looking at how quickly magnesium reacts with dilute hyrdrochloric acid.

7. Investigate the reactions of carboxylic acids
A carboxylic acid is one which contains -COOH as its functional group. In comparison to mineral acids, carboxylic acids show some similarities, but also, some important differences.

8. Titration
Determine the reacting volumes of solutions of acid and alkali by titration and determine the concentration of solutions of acid and alkali by titration.

9. Investigate the reaction of gases
Investigate the preparation, properties, tests and reactions of the gases hydrogen, oxygen and carbon dioxide.

Links
- External linkExternal link
- External linkExternal link
- SubscriptionSubscription
- External linkExternal link
- External linkExternal link
- External linkExternal link
- SubscriptionSubscription
- External linkExternal link