Exam practice
GCSE Chemistry: exam-style quiz by topic
Try this quiz based on GCSE Chemistry past papers. Choose the topic you would like to revise and answer the questions.
GCSE Chemistry: exam-style questions
Edexcel Foundation and higher GCSE interactive tests based on past papers to get you ready for your chemistry exams. Topics include the periodic table, equations and more.

GCSE Chemistry: quick-fire questions
Use our interactive quiz to understand how the Edexcel foundation and higher chemistry GCSE exams work. Revise topics such as the periodic table and equations.

Quizzes
QUIZ: Pure substances and mixtures
This interactive quiz is for GCSE Chemistry (single science) students studying pure substances and mixtures. Test your knowledge of filtration, crystallisation and distillation.

QUIZ: Atomic structure
This interactive quiz is for GCSE Chemistry (single science) students studying atomic structure. Test your knowledge of atomic models, atomic number and calculating atomic mass.
QUIZ: Groups in the periodic table (activity 1)
his interactive quiz is for GCSE Chemistry (single science) students studying the periodic table. Test your knowledge of where the different groups are in the periodic table.
QUIZ: Groups in the periodic table (activity 2)
This interactive quiz is for GCSE Chemistry (single science) students studying the periodic table. Test you knowledge of chemical and physical properties and chemical reactions.
QUIZ: Reactions of metals
This interactive quiz is for GCSE Chemistry (single science) students studying the reactions of metals. Test your knowledge of the reactivity series and the extraction of metals.

QUIZ: Acids, alkalis and salts (1)
This interactive quiz is for GCSE Chemistry (single science) students studying acids, alkalis and salts. Test your knowledge on neutralisation, reactions with acids and solutions.

QUIZ: Acids, alkalis and salts (2)
This interactive quiz is for GCSE Chemistry (single science) students studying acids, alkalis and salts. Test your knowledge of indicators, reactions and chemical equations.

QUIZ: Electrolysis
This interactive quiz is for GCSE Chemistry (single science) students studying Electrolysis. Electrolysis involves using electricity to break down electrolytes to form elements.
QUIZ: The three states of matter
This interactive quiz is for GCSE Chemistry (single science) students studying the three states of matter. Test your knowledge on the properties of substances and changes of state.

QUIZ: Ionic compounds
This interactive quiz is suitable for GCSE Chemistry (single science) students studying ionic compounds. An ionic compound is made up of charged particles, called ions.
QUIZ: Small molecules
This interactive quiz is suitable for GCSE Chemistry (single science) students studying small molecules. Test you knowledge of covalent bonds, electrons and atoms.

QUIZ: Giant covalent molecules
This interactive quiz is for GCSE Chemistry (single science) students studying giant covalent molecules.Giant covalent substances have many atoms joined together by covalent bonds.

QUIZ: Nanoscience
This interactive quiz is for GCSE Chemistry (single science) students studying nanoscience. Answer questions about nanoparticles and the properties of Nanoparticulate materials.
QUIZ: Calculations in chemistry
This interactive quiz is for GCSE Chemistry (single science) students studying calculations in chemistry. Test your knowledge of calculating relative formula mass.

Podcasts
Atomic structure and the periodic table
Learn about atomic structure and the periodic table for your GCSE chemistry exam, with Dr Sunayana Bhargava and Tulela Pea.

Bonding, structure and properties
Learn about bonding, structure and properties of matter for your GCSE chemistry exam, with Dr Sunayana Bhargava and Tulela Pea.

Chemical changes
Dr Sunayana Bhargava and Tulela Pea take you through what you need to know about chemical changes for your GCSE chemistry exam.

Science exam techniques
Learn all about science exam techniques for your GCSE science exams with Dr Alex Lathbridge.

Key concepts in chemistry
Equations and formulae - Edexcel
Chemists use symbols and formulae to represent elements, ions and compounds. Word equations and balanced chemical equations model the changes that happen in chemical reactions.

Hazards and risks - Edexcel
Hazard symbols warn about the dangers of a substance. Risk is the chance that a hazard will cause harm. Risk assessments describe how to reduce the risk of harm when carrying out an experiment.

Atomic structure - Edexcel
Atoms consist of a nucleus containing protons and neutrons, surrounded by electrons in shells. The numbers of particles in an atom can be calculated from its atomic number and mass number.
The periodic table - Edexcel
Mendeleev made an early periodic table. In the modern periodic table, elements are in order of atomic number in periods and groups. Electronic configurations model how electrons are arranged in atoms.

Ionic compounds - Edexcel
When a metal element reacts with a non-metal element an ionic compound is formed. They have a giant lattice structure with strong ionic bonds. A lot of energy is needed to overcome these bonds.

Simple molecular substances - Edexcel
A covalent bond is a shared pair of electrons. Covalent bonding results in the formation of molecules. Simple molecular substances have low melting and boiling points, and do not conduct electricity.
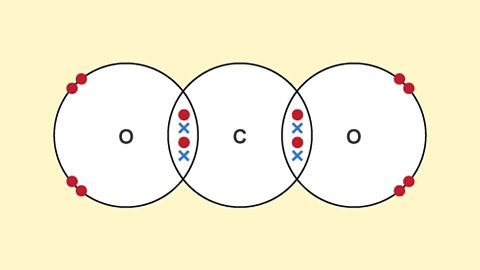
Giant covalent substances - Edexcel
Giant covalent substances contain atoms joined together by bonds. Diamond, graphite and graphene are forms of carbon and have different properties because they have different structures.

Metals and non-metals - Edexcel
Metals and non-metals have different properties. Properties of metals can be explained in terms of metallic structure and bonding. Different chemical models have different features and limitations.

Chemistry calculations - Edexcel
An empirical formula of a substance is found using the masses and relative atomic masses of the elements it contains. The law of conservation of mass applies to closed and non-enclosed systems.
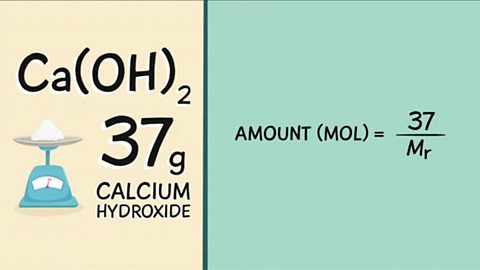
Mole calculations (higher) - Edexcel
The mole is the unit for the amount of substance. The number of particles in a substance can be found using the Avogadro constant. The mass of product depends upon the mass of limiting reactant.
Sample exam questions - key concepts in chemistry - Edexcel
Understanding how to approach exam questions helps to boost exam performance. Question types will include multiple choice, structured, mathematical and practical questions. Arrangements for exam/non-exam assessments for students taking qualifications during the pandemic may be subject to change. Please check with your teacher.

States of matter and mixtures
States of matter - Edexcel
The three states of matter can be represented by the particle model. This model explains the properties of substances in their different states, as well as changes of state.
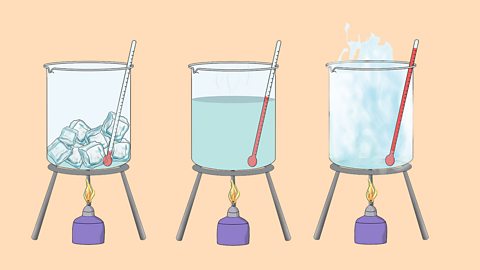
Separation and purification - Edexcel
There are different ways to separate mixtures, for example by filtration, crystallisation, distillation or chromatography. The method chosen depends upon the type of mixture.

Sample exam questions - states of matter and mixtures - Edexcel
Understanding how to approach exam questions helps to boost exam performance. Question types will include multiple choice, structured, mathematical and practical questions.

Chemical changes
Acids and alkalis - Edexcel
Indicators are used to determine if a solution is acidic or alkaline. Acids react with metals, bases and carbonates to produce salts. Neutralisation is the reaction between an acid and a base.

Salts - Edexcel
Soluble salts can be made by reacting acids with soluble or insoluble reactants. Titration must be used if the reactants are soluble. Insoluble salts are made by precipitation reactions.

Electrolysis - Edexcel
Electrolysis involves using electricity to break down electrolytes to form elements. The products of electrolysis can be predicted for a given electrolyte. Copper can be purified using electrolysis.

Sample exam questions - chemical changes - Edexcel
Understanding how to approach exam questions helps to boost exam performance. Question types will include multiple choice, structured, mathematical and practical questions.

Extracting metals and equilibria
Obtaining and using metals - Edexcel
The reactivity series shows metals in order of reactivity. The reactivity of a metal can be worked out by studying its reactions. Iron and aluminium are extracted from their ores in different ways.

Reversible reactions and equilibria - Edexcel
Chemical reactions are reversible and may reach a dynamic equilibrium. The direction of reversible reactions can be altered by changing the reaction conditions. Ammonia is made by the Haber process.

Sample exam questions - extracting metals and equilibria - Edexcel
Understanding how to approach exam questions helps to boost exam performance. Question types will include multiple choice, structured, mathematical and practical questions.

Separate chemistry 1
Transition metals, alloys and corrosion - Edexcel
Transition metals have high melting points and densities, form coloured compounds and act as catalysts. Rusting can be prevented by keeping oxygen and water away, and by sacrificial protection.

Percentage yield, atom economy and gas calculations - Edexcel
The percentage yield shows how much product is obtained compared to the maximum possible mass. The atom economy of a reaction gives the percentage of atoms in reactants that form a desired product.

More chemical calculations - Higher - Edexcel
Calculations involving the mole can be used to determine unknown concentrations, volumes and masses in reactions. One mole of any gas occupies 24 cubic decimetres at room temperature and pressure.

Fertilisers and chemicals - Edexcel
Fertilisers may contain nitrogen, phosphorus and potassium compounds to promote plant growth. Ammonium nitrate and ammonium sulfate are salts that can be used as fertilisers.

Industrial chemical reactions - Higher
The conditions chosen for an industrial reaction are related to producing an acceptable yield in an acceptable time. Different reaction pathways are often available to produce a given product.

Chemical cells and fuel cells - Edexcel
A chemical cell produces a voltage until one of the reactants is used up. In a hydrogen-oxygen fuel cell, hydrogen and oxygen are used to produce a voltage, and water is the only product.

Sample exam questions - Edexcel
Understanding how to approach exam questions helps to boost exam performance. Question types will include multiple choice, structured, mathematical and practical questions. Arrangements for exam/non-exam assessments for students taking qualifications during the pandemic may be subject to change. Please check with your teacher.

Groups in the periodic table
Group 1 - the alkali metals - Edexcel
The group 1 elements are all soft, reactive metals with low melting points. They react with water to produce an alkaline metal hydroxide solution and hydrogen. Reactivity increases down the group.

Group 7 - the halogens - Edexcel
The group 7 elements are all reactive non-metals. They react with metals to form metal halides, and with hydrogen to form acidic hydrogen halides. Reactivity decreases down the group.
Group 0 - the noble gases - Edexcel
The group 0 elements, the noble gases, are all unreactive non-metal gases. They show trends in their physical properties. Their uses depend on their inertness, low density and non-flammability.

Sample exam questions - groups in the periodic table - Edexcel
Understanding how to approach exam questions helps to boost exam performance. Question types will include multiple choice, structured, mathematical and practical questions. Arrangements for exam/non-exam assessments for students taking qualifications during the pandemic may be subject to change. Please check with your teacher.

Rates of reaction and energy changes
The rate of reaction - Edexcel
The greater the frequency of successful collisions between reactant particles, the greater the reaction rate. Temperature, concentration, pressure and the use of catalysts affect reaction rate.

Heat energy changes in chemical reactions - Edexcel
Exothermic reactions in solution give out energy and the temperature increases, while endothermic reactions take in energy and the temperature decreases. Bonds are broken and made in reactions.
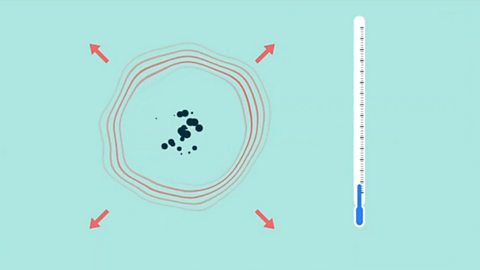
Sample exam questions - rates of reaction and energy changes - Edexcel
Understanding how to approach exam questions helps to boost exam performance. Question types will include multiple choice, structured, mathematical and practical questions.

Fuels and Earth science
Fuels - Edexcel
Crude oil is a finite resource. Petrol and other fuels are produced from it using fractional distillation and cracking. Combustion products may cause environmental and health problems.

Earth science - Edexcel
There is evidence that the Earth's early atmosphere contained less oxygen but more carbon dioxide and water vapour than it does today. Increased emissions of greenhouse gases have led to climate change.

Sample exam questions - fuels and Earth science - Edexcel
Understanding how to approach exam questions helps to boost exam performance. Question types will include multiple choice, structured, mathematical and practical questions.

Separate chemistry 2
Tests for ions - Edexcel
Flame tests and chemical tests are used to detect and identify ions in samples. Instrumental methods of analysis are faster, and more accurate and more sensitive than simple chemical tests.

Hydrocarbons - Edexcel
The alkenes form a homologous series of unsaturated hydrocarbons. Bromine water is decolourised by alkenes but not by alkanes.

Polymers
Addition polymers are made from molecules containing C=C bonds. Polymers have different properties and uses but it is difficult to dispose of them. DNA, starch and proteins are biological polymers.

Alcohols and carboxylic acids - Edexcel
Alcohols contain the 鈥揙H functional group. Ethanol is made from sugars by fermentation, and concentrated using fractional distillation. Carboxylic acids contain the 鈥揅OOH functional group.

Nature and uses of nanoparticles - Edexcel
Nanoparticles are 1 nm to 100 nm in size. They have very large surface area to volume ratios. The properties of nanoparticulate substances are different from those of the same substance in bulk.
Bulk materials - Edexcel
Materials include glass and clay ceramics, polymers, metals and composite materials. They have different physical properties, which make them suitable for different uses.

Sample exam questions - Separate chemistry 2 - Edexcel
Understanding how to approach exam questions helps to boost exam performance. Question types will include multiple choice, structured, mathematical and practical questions.

Practical skills
Practical skills
Scientific investigations have several stages - planning, collecting data, analysing data and evaluation. It is important to understand how to carry out each stage of the investigation.

Links
- External linkExternal link
- External linkExternal link
- SubscriptionSubscription
- External linkExternal link
- External linkExternal link
- External linkExternal link
- SubscriptionSubscription
- External linkExternal link