Atomic structure and the periodic table
Atoms, elements and compounds - AQA
Chemists use symbols and formulae to represent elements and compounds. Word equations and balanced chemical equations represent the changes that happen in chemical reactions.
Mixtures - AQA
There are different ways to separate mixtures, such as filtration, crystallisation, simple distillation, fractional distillation and chromatography. The method chosen depends on the type of mixture.

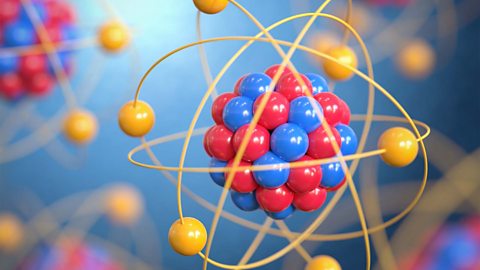
Atomic structure - AQA
Atoms consist of a nucleus containing protons and neutrons, surrounded by electrons in shells. The number of subatomic particles in an atom can be calculated from the atom's atomic number and mass number.
The periodic table - AQA
Mendeleev made an early periodic table. In the modern periodic table, elements are in order of atomic number in periods and groups. Electronic structures model how electrons are arranged in atoms.

Groups in the periodic table - AQA
Elements in the same group of the periodic table show trends in physical properties, such as boiling point. They have the same number of electrons in their outer shell, so they are similar in their chemical properties.

Transition metals - AQA
The transition elements are metals. They have high melting points and densities, and are strong and hard. They form coloured compounds and act as catalysts.

Sample exam questions - atomic structure and the periodic table - AQA
Understanding how to approach exam questions helps to boost exam performance. Question types will include multiple choice, structured, mathematical and practical questions.

Links
- External linkExternal link
- External linkExternal link
- SubscriptionSubscription
- External linkExternal link
- External linkExternal link
- External linkExternal link
- SubscriptionSubscription
- External linkExternal link