Atomic and nuclear physics
The structure of the atom and the nucleus - CCEA
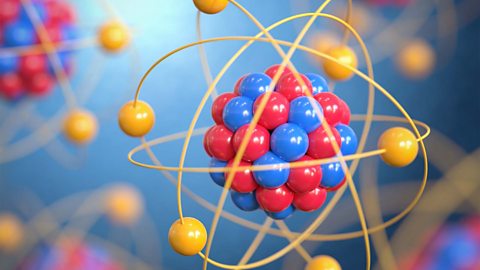
What is the structure of an atom? Well, atoms are made up of protons, neutrons and electrons. Change the number of neutrons in an atom and it becomes an isotope, change the number of electrons, it becomes an ion! Learn more about atoms and the Rutherford-Bohr model of an atom in this CCEA Double Award revision guide.
Radioactive decay and half-life - CCEA
Radioactivity was first noticed by French physicist, Henri Becquerel, in 1896, when he observed that some photographic plates which had been stored close to a uranium compound had become partly exposed or âfoggedâ.

The dangers and uses of radiation - CCEA
People are exposed to sources of radiation in all aspects of everyday life. Radioactive sources can be very useful but need handling carefully to ensure safety.

Nuclear fission - CCEA
Nuclear fission is the splitting of a large atomic nucleus such as uranium into smaller nuclei with the release of energy.

Nuclear fusion - CCEA
Nuclear fusion occurs when two small, light nuclei join together to make one heavier nucleus. The nuclei fuse together, and energy is released.

Links
- External linkExternal link
- External linkExternal link
- SubscriptionSubscription
- External linkExternal link
- External linkExternal link
- SubscriptionSubscription
- External linkExternal link
- External linkExternal link
- SubscriptionSubscription