Chemical patterns
How have our ideas about atoms changed over time? - OCR 21st Century
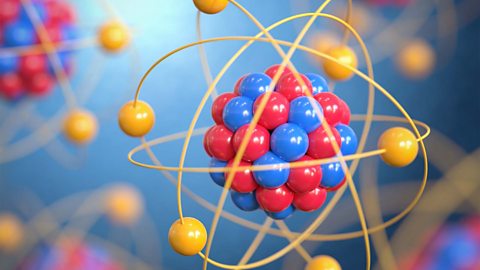
The atomic model consists of a nucleus containing protons and neutrons, surrounded by electrons in shells. The numbers of particles in an atom can be calculated from its atomic number and mass number.
What does the periodic table tell us about the elements?
Mendeleev made an early periodic table. In the modern periodic table, elements are arranged in order of atomic number in periods and groups. Electronic arrangements model how electrons are arranged.

How do metals and non-metals combine to form compounds?
The group 0 elements, the noble gases, are all unreactive non-metal gases. They show trends in their physical properties. Their uses depend on their inertness, low density and non-flammability.

How are equations used to represent chemical reactions?
All substances are described by their formulae, which are used to write balanced chemical equations. Writing the formula for an ionic compound requires knowledge of the charges on its ions.

Links
- External linkExternal link
- External linkExternal link
- SubscriptionSubscription
- External linkExternal link
- External linkExternal link
- SubscriptionSubscription
- External linkExternal link
- External linkExternal link
- SubscriptionSubscription