Rate of chemical change
Rates of reaction
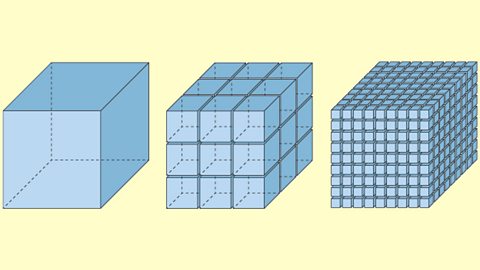
Chemical reactions vary in speed. The rate of reaction measures how much product is made in a given time. For reactions to occur, reactant particles must collide.

Factors that affect the rate of reaction
Chemical reaction rates increase or decrease according to factors including temperature, pressure and light. A catalyst changes the rate of reaction but is unchanged at the end of the reaction.
Links
- External linkExternal link
- External linkExternal link
- SubscriptionSubscription
- External linkExternal link
- External linkExternal link
- External linkExternal link
- SubscriptionSubscription
- External linkExternal link