Chemical reactions
Introducing chemical reactions - OCR Gateway
Chemists use symbols and formulae to represent elements, ions and compounds. Chemical equations model the changes that happen in chemical reactions.

Avogadro constant and moles - OCR Gateway
The mole is the unit for amount of substance. The number of particles in a substance can be found using the Avogadro constant. The mass of product depends upon the mass of limiting reactant.

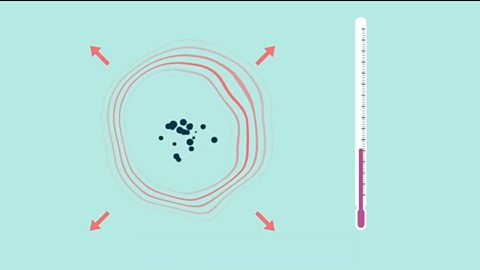
Energetics - OCR Gateway
Exothermic reactions in solution give out energy and the temperature increases, while endothermic reactions take in energy and the temperature decreases. Bonds are broken and made in reactions.
Types of chemical reactions - OCR Gateway
Oxidation is the gain of oxygen and reduction is the loss of oxygen. Neutralisation is the reaction between an acid and a base. Acids react with metals, bases and carbonates to produce salts.

Electrolysis - OCR Gateway
Electrolysis involves using electricity to break down electrolytes to form elements. The products of electrolysis can be predicted for a given electrolyte. Copper can be purified using electrolysis.

Links
- External linkExternal link
- External linkExternal link
- SubscriptionSubscription
- External linkExternal link
- External linkExternal link
- SubscriptionSubscription
- External linkExternal link
- External linkExternal link
- SubscriptionSubscription